Neuroanatomy and Physiology of
the “Brain Reward System”
in Substance Abuse
I. Introduction
How does experimental use of
substances of abuse lead to drug addiction in some individuals? How do these drugs cause intoxication? Part of the answer lies in a common reinforcement
pathway in the human brain which drugs of abuse stimulate, potentially leading
to addiction (1,2,3,4,7). This reinforcement pathway, which is composed of both
central nervous system structures and endogenous neurotransmitters
communicating between these structures, has been termed the “reward
pathway”(1). The reward pathway evolved
to promote activities that are essential to the survival of the human race as
well as other mammals.
One may compare the mechanism of
drugs of abuse with that of viruses.
Viruses and drugs of abuse are both foreign to humans. Viruses enter an animal’s cells and use the
pre-existing cell “machinery” to synthesize more viruses, thus promoting their
own survival. As the viruses infect
more and more cells, the organism may become ill. Illicit drugs can take advantage of an organism in a similar
fashion. Just as viruses take over cell function throughout the body, drugs of
abuse modify cell function in these important brain structures leading to
modifications in behavior. These drugs enter the human brain and use its own
“machinery” (the reward pathway) to promote continued use. Just as the cell’s survival is dependent on
its “machinery” so is the survival of the organism dependent on an intact brain
reward pathway. Drugs of abuse,
although harmful to the organism, are able to capture this “machinery “ in some
individuals driving further drug use.
Depending on our own characteristics
(our inherited neurochemical make-up etc.) we may be more susceptible to the
illness of drug addiction just as certain people are more susceptible to infection. Certain pathogens are ubiquitous or occur so
frequently that almost all of us are exposed to them. Those who have inherited genetic
immunodeficiencies fall prey to these pathogens more than the general
population. Similarly, individuals who
have a genetic predisposition, may be more vulnerable to addiction after
exposure to the drug.
As noted earlier, substances of
abuse affect the brain reward pathway, which is made of neurons that release
chemicals when they are stimulated.
This release leads to subjective feelings of well being (1,2). This brain reward system evolved to subserve
activities essential to species survival, such as sexual activity and feeding
behaviors. Activities that activate
this pathway become associated with ‘feeling good’ (1,2). For example, sexual intercourse causes
release of chemicals activating this pathway, and the result is a feeling of
well being. Thus, the reward pathway
serves to promote survival of the species by rewarding behaviors necessary for
continued survival (seeking food, reproduction, shelter, drink, etc). Drugs of abuse stimulate this “brain reward”
pathway in a similar fashion, and this is why substance users experience
feelings of pleasure or “high” when they use them (1,2). When drugs of abuse are repeatedly used,
they may “commandeer” the brain reward system, driving compulsory drug use to
the exclusion of other adaptive activities.
Thus, “addiction” can be partially explained by the action of drugs of abuse
on this common reward pathway, in which drug use stimulates further use and
drug seeking behavior (1,2,3,4).
In the following paragraphs, the
basic anatomy of the reward pathway and brain structures that interact with
this pathway will be discussed. Next,
the molecular physiology of the reward pathway will be delineated. After laying
the foundation of the anatomy and physiology of brain reward, the specific
interactions of drugs of abuse will be examined. Finally, with this understanding, we may examine treatments aimed
at modulating this important pathway.
II. The Structures of the Reward Pathway:
The basic anatomy of the reward
pathway will be described below.
However, it must be remembered that the anatomical structures involved
have complex interrelationships, and are modulated by other parts of the brain
and other neurochemicals. Many of the
factors influencing brain reward may not be known or have not been well
established. Therefore, this paper provides
an overview of the most well established structures and pathways involved. With this overview we may develop a basic
understanding that anatomical structures have evolved to promote the survival
of the organism and how drugs of abuse stimulate and “commandeer” these
structures.
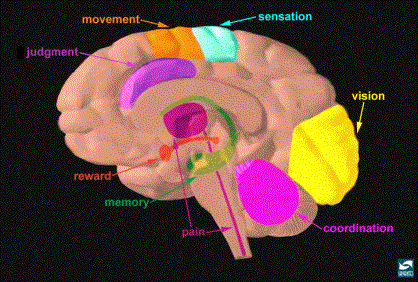
Core
Structures of the Reward System
The core structures of the brain
reward pathway is located in the limbic system, a set of primitive structures
in the human brain (1,2,3,7,9).
Conceptually, the function of the limbic system is to monitor internal homeostasis,
mediate memory, mediate learning, and experience emotion. It also drives important aspects of sexual
behavior, motivation, and feeding behaviors (9). The primary nuclei (or parts) of the limbic system include the
hypothalamus, amygdala, hippocampus, septal nuclei, and anterior cingulate
gyrus (9). Also important in the
function of the limbic system is the limbic striatum, which includes the
nucleus accumbens, ventral caudate nucleus and the putamen (9). The nucleus accumbens (NA) has been
implicated as an especially important structure of the brain reward pathway
because drugs of abuse target it. (1,2,3,5,7). Other structures important in brain reward include the amygdala
and the ventral tegmental area (VTA). (1,2,3). The majority of this paper will concentrate
on the NA and the VTA.
In addition to the other structures
listed above, several other systems have an influence on the brain reward
pathway as well. The endocrine and the
autonomic nervous systems interact via the hypothalamus, an integral part of
the limbic system, and the pituitary (1).
These structures modulate the reward pathway. The hypothalamus is involved in every aspect of endocrine,
visceral, and autonomic functions, and it is able to influence eating,
drinking, sexual activity, aversion, rage, and pleasure (1,9). Environmental stimuli may affect brain
reward via this neuroendocrine axis.
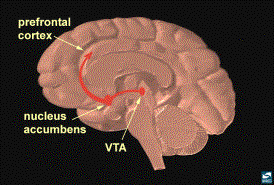
Electrical Stimulation of the Brain Reward Pathway
The link between the brain reward
system and the hypothalamic-pituitary axis may be seen through experiments on
animals where electrodes are placed into the nucleus accumbens (part of the
brain’s reward system) under conditions of imposed environmental stress. Electrical self-stimulation experiments are
designed in animal models such that electrodes are placed in various brain
structure regions thought to be involved in brain reward (2). The animal then performs an activity (i.e.
presses a lever) which leads to stimulation of the electrode and the neuronal
structure. If the electrode is placed
in a structure in the reward pathway, the resulting stimulation is pleasurable
and self-stimulation is therefore encouraged. In models of addiction hungry animals
will demonstrate a preference for self-stimulation over food and drink to the
point of starvation (2). This
experimental paradigm shows how drugs of abuse can commandeer this system and
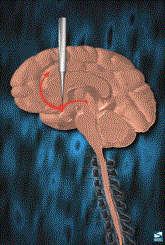
become even more rewarding than the behaviors it evolved to subserve. Other techniques allow the animal to press
a lever and the drug is injected into specific areas of the brain as
illustrated below. If the point of
injection is not within the brain reward pathway, reinforcement will not occur. Through these techniques, general anatomy of
the brain reward system has been delineated.
Experimental Self-Stimulation of the Brain Reward Pathway

Core Structures of
the Reward System
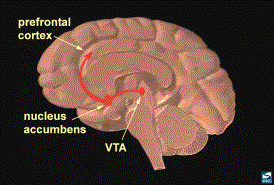
As discussed before the reward
pathway, located in the limbic system, is primarily made up of core structures
that are connected by the median forebrain bundle (MFB): the nucleus
accumbens (NA), the ventral tegmental area (VTA), the ventromedial
and lateral nuclei of the hypothalamus, and the amygdala (1,2,3,7). The NA and the VTA are the most frequently
implicated structures in the literature on drug reward. The MFB is composed of
nerves that connect the septum, amygdala, the NA and the olfactory tubercle
with the hypothalamus and the VTA (1,9).
In other words, the MFB is like a power line of neurons connecting the
structures of the reward pathway with other brain structures. Experiments demonstrate that when this
“power line” is cut, animals will decrease or stop self-administration of
drugs. Thus, an intact MFB appears to
be necessary for the “brain reward” system to function properly. Below are slides that show the anatomy of
neurons and the electrical activity that occurs between them.
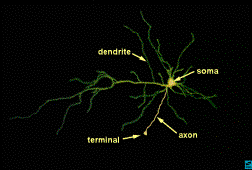
NEURON AND NEURON
FUNCTION
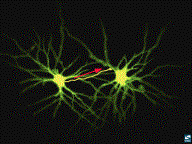
ELECTRICAL ACTIVITY BETWEEN TWO
NEURONS
Dopaminergic neurons making up the
MFB or “power line” of the brain reward system run from the VTA to other
structures involved in brain reward.
The neurotransmitter they release is called dopamine. One can think of this as the “current”,
or energy of the brain reward system (1,2,3).
2.2 Communicating Brain Structures
The central reward pathway of the
brain sends information to and receives input from many other brain structures:
the reticular activating system (RAS), the central gray around the aqueduct of
Sylvius, limbic regions, and the basal ganglia and cerebellum. Located in the brain stem, the RAS controls
attention and arousal to various sensory inputs from our environment. Limbic regions such as the amygdala, the
septum and the thalamus provide input to the reward pathway concerning
motivational and emotional variables.
Next, the reward pathway interacts with the basal ganglia and cerebellum
to modify motor activity. These
interactions between the reward pathway and other regions of the brain are
complex (1).
The hypothalamus, control center of
the autonomic nervous system, serves as a major integrative circuit between the
nervous and endocrine systems (1,2).
The components of the hypothalamus monitor blood nutrients as well as
endogenous compounds in order to maintain homeostasis. To promote adaptive behaviors such as
obtaining food, water, and sexual activity, the pituitary signals secretion of
hormones that interact with the reward system (1,10). Thus, the hypothalamus is the command center interconnecting the
reward pathway with the body and environmental stimuli. These stimuli are analogous to a complex
array of music each a melody of summation in the form of endocrine hormones,
blood temperature, blood osmolarity, blood volume, and neurotransmitters are
deciphered and directed by the hypothalamus.
This command center then sends out signals in the form of
neurotransmitters (chemical signals) to various parts of the brain including
the brain reward system.
Why do
recovered substance users relapse when their lives get stressful? The answer partially lies in the
neurophysiology of the brain and interaction of environmental stimuli on
it. Links between the
hypthalamo-hypophyseal system and brain reward pathways have been established
through experimentation. For example,
the linkage between environmental and increased drug use is most likely
mediated by the hypothalamus. Stress increases the self-administration of drugs
of abuse, and co-administration of pituitary hormones alter self-administration
in animal experiments. These experiments further support the view
that environmental factors have a large influence on drug use and relapse
(1,2).
III. Genetic and Biological evidence for a
Common Reward Pathway
3.1 Evolution of the Pathway
As noted earlier, the intrinsic
purpose of an endogenous reward center is to reinforce behaviors that promote
the survival of a species. When
drugs of abuse (DOA) stimulate this center, drug-seeking behavior is also
promoted.
3.2 Genetics and Population Studies
Why do studies show that many individuals experiment with DOA but
only some become addicted? Genetic
studies in both animals and humans have contributed to our ability to answer
this question. Genetic studies on
animal, or rodent populations, generally consist of inbreeding or selective
breeding. Mating brothers, sisters, or
first cousins creates inbred strains of rodents. After 20 generations of mating a strain is created that is, for
all practical purposes, genetically identical (1). The next step is to look at differences in response to DOA
through experimentation on different rodent strains.
In selective breeding, a desirable
trait is chosen. For example,
researchers may breed for a high response to EtOH. Rodents who have the desirable trait are then mated. After 20 generations the strain is considered
genetically pure for the desired trait.
Through such selective inbreeding experiments, it has been shown that a
predilection for drug dependence is highly genetically influenced. Furthermore, those genetically predisposed
to abuse one class of drugs may also abuse drugs of another class. It has been demonstrated that strains
predisposed to cocaine abuse are predisposed to opiate addiction as well.
Human studies investigating the
genetics of drug abuse consist of either adoption or twin studies (1). Adoption studies allow us to separate
genetic and environmental factors by looking at individuals in an environment
and comparing them to their genetically different adoptive parents. The individuals are then compared to their
biological parents. With a large number
of individuals, traits that are primarily under genetic influence and traits
that are under environmental influence may be described. In twin studies, identical and fraternal
twins are compared. Identical twins
share 100% of their genes while fraternal twins share 50% of their genes. The relative agreement of behavioral traits
to these percentages suggests the proportion of genetic and environmental
influences. Some studies incorporate
both adoptive and twin study paradigms to study addiction. Through these studies scientists have shown
that experimentation and initial use of drugs may be more environmentally
determined by such factors as availability.
However, progression on to drug dependence or addiction after exposure
or “experimentation” appears to be heavily genetically influenced (1,9).
IV. Molecular Physiology of the Reward Pathway
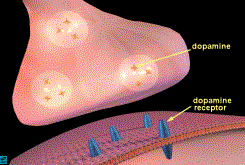
Dopamine Binding at the Neuron Level
The primary neurotransmitter of the
reward pathway is dopamine (1,2,3,4).
Although drugs of abuse often act through separate mechanisms and on
various locations in the brain reward system, they share a final common action
in that they increase dopamine levels in the brain reward system. However, neurotransmitter systems are
inextricably intertwined. Thus
serotonin, endogenous opiates, as well as GABA also modulate dopamine levels in
the brain reward pathway (1,2,3). In
the following paragraphs the major neurotransmitters involved in brain reward
will be discussed.
4.1 Dopamine
Drugs of abuse have been shown to
increase dopamine neurotransmitter levels in the reward pathway
(1,2,3,4). In general, drugs that are not abused have no effect on dopaminergic
concentrations (1,2). Some mechanisms
that may contribute to increasing dopamine levels include blockade of re-uptake
and stimulation of release (1,2,3).
The specific mechanisms of action of various substances of abuse in
increasing dopamine levels in the brain reward pathway will be described
below.
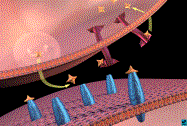
Neurotransmitter Release and
Re-uptake
4.2 Other Neurotransmitters and Brain Reward
4.2a Serotonin
Even
though increased dopamine in the brain reward system is generally thought to be
the final common pathway for the reinforcing properties of drugs, other
neurotransmitters such as serotonin are involved in the modulation of both drug
self-administration and dopamine levels.
Serotonin may be important in modulating motivational factors, or the
amount of work and individual is willing to perform to obtain a drug (1).
Serotonergic neurons project both to the NA and VTA and appear to regulate
dopamine release at the NA. However the
relationship between serotonin and dopamine release is complex in that, serotonin
has numerous receptor types and its regulation of dopamine release is at times
inhibitory and at other times excitatory (1,2). Thus, serotonin modulates the reward pathway through various
mechanisms by interacting with different receptors throughout the brain.
4.2b GABA
GABA, another neurotransmitter
involved in the modulation of dopaminergic reward systems, plays a role in the
mediation of effects of many drugs of abuse (1,2,3). GABA is an inhibitory neurotransmitter located diffusely
throughout the brain. Drugs of abuse
(DOA) act on the GABA receptor to hyperpolarize neurons. When a neuron is hyperpolarized, it is
inhibited from firing. An analogy may
be applying brakes to a car. Just as
greater amounts of gas are required to cause the car to move while stepping on
the brakes, greater amounts of stimuli are required to cause a neuron to fire
that is hyperpolarized. When neurons
fire they release neurotransmitter, and since drugs of abuse (DOA) inhibit these
neurons, they release less GABA. When
barbiturates, benzodiazapines or alcohol interact with the GABA receptor, they
inhibit the release of GABA onto the dopaminergic neurons (1,2,3). Thus, this is like taking your foot off the
brakes & allowing the car to go full speed ahead. The net result is disinhibition of dopaminergic neurons, making
them fire more rapidly and releasing more dopamine in the reward system. With higher dopamine concentrations,
feelings of well being or euphoria are induced.
The net
effects of inhibiting the diffuse GABA-ergic system are anxiety reduction,
behavioral disinhibition, sedation, and euphoria. Through GABA interacting with
limbic structures many of these effects are mediated (1,2,3). Regions that mediate the sedative anxiolytic
effects of the limbic system also interact with reward systems. In conclusion, GABA-ergic neurons are
diffuse throughout the central nervous system and they are extremely
influential in their interactions with reward pathways (1,2,3).
4.2c The Endogenous Opiates
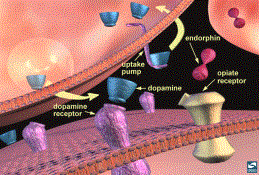
Endorphin Binding at the Synapse
Just as the structures of the brain
reward system encourage adaptive behaviors such as seeking food and sex,
endogenous proteins called endorphins also motivate behaviour (1,3). The
“runner’s high” is thought to be related to the production of such endogenous opiate compounds or endorphins. In addition, place preference (animals
prefer the environment where the drug is administered to other environments) is
elicited when endorphins are applied to the NA (1,2). Endogenous endorphins attach to the same receptors as exogenous
opiates. Through the same mechanism,
they both increase dopamine in the brain reward pathway (1,2,3).
5. Drugs of Abuse and the Reward Pathway
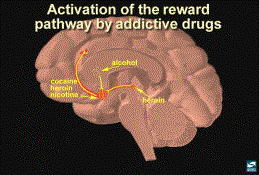
Primary Location of Action for Drugs
of Abuse
Experiments have shown that specific drugs of abuse affect
receptors/neurochemical response act in different areas of the brain. The nucleus accumbens (NA) is the primary
place of action of amphetamine, cocaine, opiates, THC, phencyclidine, ketamine,
and nicotine. Opiates, alcohol,
barbiturates and benzodiazapines (2,3,4) stimulate neurons in the ventral
tegmental area (VTA). The final common action of most substances
of abuse is stimulation of the brain reward pathway by increasing
dopamine. The action of drugs of abuse
on structures of the reward pathway at the gross anatomical level, followed by
molecular actions of this system, will be discussed below.
5.1 Alcohol
The complete discussion of the
anatomical and biochemical effects of alcohol is beyond the scope of this
paper. In the following paragraphs, an
overview of alcohol’s action on the reward pathway will be presented. Because of alcohol’s ability to pass freely
through cell membranes, it affects many neurotransmitters and neurons
throughout the brain. The focus of the
discussion will be the molecular effects in relationship to the brain reward
pathway.
Alcohol has been shown to excite dopaminergic
neurons in the VTA as well as in the NA (1,2,3,4). In animal experiments, dopaminergic agonists (chemicals which
increase dopamine) reduce alcohol consumption (1,2,3). For example, the dopamine agonist
bromocriptine increases water intake and decreases consumption of alcohol in
rodents(1). Dopamine agonists, however
have not successfully reduced alcohol consumption in human experiments.
Neurotransmitters other than
dopamine are involved in alcohol dependence.
Studies demonstrated that serotonin re-uptake inhibitors (medications
that are commonly prescribed for depression such as fluoxetine ) may decrease
consumption of alcohol in both rodents and in humans (2). It is not known whether this is due to a
direct or an indirect effect of serotonin on dopaminergic systems, or a
combination effect. Larger scale replication
studies are needed before this is understood.
The effects of serotoninergic medications on drug self-administration
may be due to their effects on motivational factors, as opposed to the specific
reinforcing effects of the drug by modulating the reinforcing properties of
other reinforcers such as food, water, alcohol and drugs of abuse (2).
5.2 Opiates
Opiate
Action in the Brain
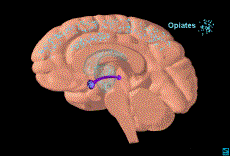
Opiates influence dopamine levels and brain reward
indirectly by inhibiting GABA neurons in the VTA (1). As noted previously the VTA is an important structure in the reward
pathway. GABA neurons inhibit
dopaminergic neurons in the VTA. Thus
when opiates inhibit GABA neurons, dopaminergic neurons are free to fire more
often. In essence, opiates remove the
“brakes” from dopaminergic neurons and allow them to fire more rapidly
resulting in an increase of dopamine release (1,2).
Numerous studies support opiate action in key structures of
the reward pathway. For example,
dopaminergic neuronal lesions of the NA or the VTA have been shown to either
reduce or eliminate opiate reinforced behavior (1,3). Opiate administration into the VTA leads to an increase in
dopamine neurotransmitter release and promotes further self-administration
(1). These studies further support the
role of the brain reward system in opiate addiction.
Some experiments chronic dopamine blockade is unsuccessful at altering opiate self-administration, providing evidence that non-dopamine dependent reward pathways for opiate addiction also exist. (1,3). However, most experiments demonstrate that opiates function by stimulating both the NA and the VTA, both of which are key structures in the reward pathway. However, as lesions to dopaminergic neurons do not completely eliminate self-administration of opiates in some experiments, indirect and dopamine independent mechanisms of opiate addiction and reinforcement also exist (3).
5.3 Stimulants
Like other drugs of abuse,
stimulants increase dopamine concentrations in the brain reward pathway
(1,2,3,4,7). Stimulants such as
cocaine, amphetamines, caffeine and nicotine all stimulate the brain reward
pathway through slightly different mechanisms and increase dopamine to
different extents. Moreover, the route
of administration of stimulants also influences the addictive properties of
stimulants, by influencing the rate at which dopamine “spikes” in the
brain. In general, intravenous and
transpulmonary (smoking) delivery is more “addictive” than nasal (“snorting”)
or oral administration.
5.3a Cocaine
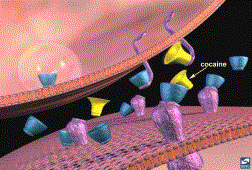
Cocaine Binding at the Synapse
Cocaine, like other drugs of abuse,
increases dopamine in the reward pathway.
Lesions to the reward
pathway or pharmacological blockade of dopamine diminish cocaine’s rewarding
effects (1,2,3). Thus, dopaminergic
neurons in the reward pathway have been shown to be important in the reinforcing
effects of cocaine (2). This increase
of dopamine in the brain reward centers is dose dependent (the more cocaine
that is taken in, the higher the dopamine concentration in these areas). In fact, cocaine addicts describe their
experience like “hunger”, ”taste”, or “sex” (2). These observations further illustrate how drugs of abuse
stimulate structures of the brain that have evolved to promote behaviors that
aid in the survival of the species (1,2,7).
Cocaine’s effects on the reward
system are so powerful that it may override other generally gratifying
reinforcers: money, safety, loved ones, morality and even survival may become
less important to the abuser than obtaining and using cocaine (2). Thus, cocaine stimulates the brain reward
system more effectively than the behaviors that the reward system evolved to
reinforce!
Cocaine acts to increase dopamine
levels by inhibiting monoamine (dopamine, serotonin, and norepinephrine)
re-uptake from the synaptic cleft (1,2,3,4,6,7). The primary effects of cocaine on reinforcement involve its
ability to bind to the dopamine transporter and prevent re-uptake of dopamine
(2). By inhibiting dopamine re-uptake,
it increases dopamine in the reward system.
5.3b Amphetamines
Like cocaine, amphetamines stimulate
the brain reward pathway by increasing concentrations of dopamine. Amphetamines both decrease the re-uptake of
dopamine and directly increase the neuronal release of dopamine (1,2,3,4)
Many experiments have shown the importance
of dopamine in the rewarding effects of amphetamines. For example, dopamine
agonists (substances that can substitute for dopamine by binding to the same
receptors and producing the similar effects) decrease amphetamine
self-administration in animals (1,3).
On the other hand, dopaminergic blockade (antagonists) decreases
amphetamine self-administration in rats by preventing amphetamine binding,
thereby preventing its dopaminergic effects (3). Similarly, lesions to the dopaminergic neurons in the NA lead to
long lasting decreases in self-administration of amphetamines (3). These examples emphasize how drugs increase
dopamine levels and stimulate the reward pathway. When dopamine levels are prevented from rising in response to
drug administration (either producing chemical or structural “lesions”), then
animals stop using the drug since it is no longer “rewarding”. These elegant experiments demonstrate that
the “rewarding” properties of the drug are directly related to whether it
increases dopamine or not in the reward pathway.
5.4 Nicotine
Nicotine is thought to affect the
brain reward system by increasing dopamine concentrations through interacting
with nicotinic acetylcholine receptors.
It has been shown to mimic endogenous (or the body’s natural)
acetylcholine neurotransmitter. Nicotine increases dopamine efflux in the
reward pathway by mimicking acetylcholine at presynaptic nicotinic receptor
sites, and exciting dopaminergic neurons (2).
Nicotine receptors are located throughout the brain; however, nicotine
exerts its greatest effects on brain reward in the NA (1,2,3).
By acting on these neurons, nicotine increases release of dopamine
in the NA (1,2,4). Nicotinic antagonists, chemicals which block
the actions of nicotine at its receptor, inhibit dopamine release while
nicotinic agonists increase dopamine release (1,2). Thus, nicotine leads to increased dopamine concentrations in the
brain reward pathway like other drugs of abuse.
5.5 Caffeine
Caffeine is the psychoactive drug
that is most commonly used throughout the world (11). Caffeine blocks the actions of adenosine, an inhibitory
neurotransmitter, by binding to its receptor and preventing post binding
changes from taking place (2). Caffeine is a “competitive antagonist” of adenosine. Since the neurotransmitter adenosine is like
the safety on a gun, when it is removed, neurons begin to fire. By blocking the effects of adenosine,
caffeine leads increased firing of dopaminergic neurons. This is especially evident in the NA (4,5).
5.6 Treatment Implications of the Brain Reward System
Operating through different
mechanisms drugs of abuse have a final common pathway by which they increase
dopamine levels within the core structures of the so called “brain reward
system” which includes the VTA and NA.
A balance between the negative effects of the drug and positive feelings
associated with stimulation of the brain reward system determine if an
individual will enjoy and continue using the substance or not (1,2). Generally the positive effects or “high” of
using a drug occur immediately or shortly after use, by the action of
increasing dopamine.
The closer positive and negative
effects are to the actual time of drug use, the more likely we are to associate
these effects with the drug.
Unfortunately, the negative consequences of drug use often come much
later and more unpredictably compared to the immediate pairing of drug
administration and reward. For example,
the later potential negative consequences of chronic drinking (such as liver
disease) may not be as important as the immediate rewarding positive effects of
drinking. Some approaches to treatment
attempt to consistently pair the negative consequences of drug administration
with drug administration. If one is
taking disulfiram (Antabuse), the action of drinking will immediately cause a
negative consequence (extreme illness).
The immediate negative consequence of drinking now competes with the
normally immediate positive reward of drinking to combat illness. By changing the time course of positive and
negative drug effects through behavioral interventions or pharmaceutical
interventions, we may be able to better treat addictions in the future.
VI. Pharmacotherapy of Drug Addiction:
Pharmacotherapeutic interventions have been developed to decrease drug use by influencing the brain reward system. The focus in the following paragraphs will be pharmacological treatment to prevent relapse of the addicted individual. General strategies for pharmacological treatment of drug addiction include creating aversion to the addicted drug, bringing consequences or punishment closer to the reinforcement of drug use, modification of neurotransmitters to decrease drug intake, and long-term substitution with a less addictive and cross-tolerant medication (1).
6.1 Aversive Conditioning
Increasing the negative or aversive effects of a drug is one effective treatment used for alcohol addiction. Disulfiram (Antabuse), metronidazole, or calcium carbimide is used to create negative effects with the ingestion of alcohol (1,2). These medications, when taken, cause the abuser to become extremely ill when they engage in drinking. Instead of experiencing the negative effects of alcohol the next day (hangover) or years later (liver disease), they experience unpleasant effects such as nausea, vomiting, and flushing in closer proximity to ingestion which opposes the normally immediate positive reward of the drug (see above). Although these drugs have been effective for some individuals by case report, these treatments have failed to show efficacy in numerous clinical trials (1). However recent work by Carroll et al demonstrates that Antabuse can be effective in preventing cocaine dependent individuals from relapsing into cocaine (12). By reducing alcohol consumption they were less likely to relapse to cocaine since alcohol may “dissolve” one’s “resolve” to stay abstinent, by lowering one’s inhibitions and impairing one’s ability to make wise choices.
6.2 Neurotransmitter Manipulation
By manipulating neurotransmitters in the reward pathway, we can potentially modify cravings for drugs of abuse. This can be accomplished in two ways. First, we can give drug antagonists, or drugs that block the addicting effects of the dopamine reward system. For example, dopamine blocking agents have been shown to diminish intake of all drugs of abuse in animal studies (2). However, application in humans has been less promising. In humans, the euphoria induced by amphetamine administration is attenuated by dopamine blocking agents. Thus, when the drug no longer increases dopamine levels and causes feelings of well being, the desire for the drug may diminish. Bupropion, a dopamine agonist, has been shown in nicotine addiction but has not been shown to be effective in cocaine addiction. Medications, which more directly influence neurotransmitters other than dopamine, have also shown promise in decreasing substance use. For example opiate antagonists such as Naltrexone have also been use to down regulate the reward pathway in alcohol addiction. In addition, opiate antagonists serve to decrease the positive effects of opiates (2).
Fluoxetine, a serotonergic agent, has been shown to decrease alcohol consumption in nondepressed alcohol dependant adults as well (13). Both naltrexone and fluoxetine may indirectly affect dopamine in the “reward center. Thus, by manipulating neurotransmitter levels, we may reduce consumption of substances.
6.3 Pharmacological Substitution
By substituting one substance that
stimulates the brain reward pathway with another less addictive/ less harmful
substance, we may aid in relapse prevention.
One example is the use of methadone to treat heroin addiction. Methadone does not have the euphoric effects
that heroin does; however, it does adequately stimulate the brain reward system
and provides a safer alternative to heroin use. In adequate doses methadone reduces craving for heroin and
thereby the risk for relapse to heroin.
Methadone, an orally administered opiate, is associated with less risk
of acquiring HIV, hepatitis C, and criminal activity - all of which are highly
associated with heroin dependence (2).
Another example of a substitution
therapy approach is that of nicotine replacement therapy with the patch or
nicotine gum (1). This allows the
individual to struggle with behavioral aspects of drug addiction and minimize
the pharmacological aspects for a time being.
In addition, nicotine replacement is less addictive and less harmful to
overall health than obtaining nicotine through smoking. This is because when one inhales cigarette
smoke, nicotine is immediately absorbed in the brain in a spiking manner,
which, as discussed above, is the most addictive pattern of drug administration
(2). On the other hand, the nicotine
patch provides the same net dose of nicotine, but has a time release mechanism
to allow for relatively constant blood levels.
Thus, the gradual increase in nicotine that occurs with the nicotine
patch will lead to more constant moderate levels of dopamine in the brain as
opposed to the “spike and dip” pattern produced by most drugs of abuse. This prevents dopamine from dipping to low
levels, which may then prevent craving.
Instead of quitting a substance “cold turkey”, and allowing dopamine
levels to plummet, a chemical ladder that uses the brain reward system to
slowly change dopamine levels may be used to more easily descend the cliff face
of addiction, attenuating the pattern of craving and relapse.
VII. Conclusion:
In the last decade it has become clear that addiction, in addition to having environmental determinants, is also of the brain. Scientists have found that a common reward pathway exists in the brain. When stimulated by drugs of abuse, addiction often occurs especially in those who are genetically or otherwise neurochemically vulnerable. This pathway, located in the primitive limbic system, has evolved over time to promote behaviors that increase the survivability of organisms, such as feeding and reproductive behaviors. Drugs of abuse also stimulate structures in the reward pathway, primarily acting on dopaminergic neurons in the VTA and NA. These drugs not only stimulate areas of the brain that have evolved to encourage adaptive behaviors; they stimulate these areas more effectively than the survival behaviors themselves! Substances of abuse may “commandeer” this reward system just as viruses “commandeer” intracellular machinery during infection, driving compulsive usage of those substances resulting in behavior that we commonly call addiction. Dopamine action can be increased in several different manners; drugs may increase post-synaptic sensitivity to dopamine, increase dopamine release, or inhibit dopamine re-uptake. Drugs of abuse may accomplish this by acting directly on dopaminergic neurons or indirectly through other neurons and neurotransmitters. Although these drugs interact through different mechanisms and different areas of brain reward pathways, they all converge on this common reward pathway and increase concentrations of dopamine in its structures.
As the mechanism of the reward pathway and its interactions with other areas in the brain become clearer, new pharmacotherapies and behavioral treatments may be developed to effectively treat substance use disorders and decrease its devastating human cost.
REFERENCES
1.Lowinson, J; Ruiz, P; Millman, R;
Langrod, J. Substance Abuse: A
comprehensive Textbook 3rd Edition.
Williams & Wilkens 1997.
2. Niesink, R; Jaspers, R; Kornet,
L; van Ree,J. Drugs of Abuse and Addiction: Neurobehavioral Toxicology. CRC
Press 1999.
Ritz, Mary. Reward Systems and
Addictive Behavior. Chapter 5, Pg124-149.
Ritz, Mary. Molecular
mechanisms of addictive substances. Chapter 6. Pg150-189.
3. Koob, G.; Nestler, E. The
Neurobiology of Drug Addiction. The
Journal of Neuropsychiatry and Clinical Neurosciences 1997; 9:482-497.
4. Bardo, Michael.
Neurophrmacological Mechanisms of Drug Reward: Beyond Dopamine in the Nucleus
Accumbens. Clinical Reviews in
Neurobiology 1998, 12(1&2): 37-67.
5. Dager, S; Layton, Matt; et
al. Human Brain Metabolic Response to
Caffeine and the Effects of Tolerance. Am J Psychiatry 156:2, Feb. 1999.
6. Childress, A; Mozley, D; et al.
Limbic Activation During Cue-Induced Cocaine Craving. Am J Psychiatry 156:1,
Jan 1999: 11-18.
7.
Little, K; Zhang, L; et al.
Striatal Dopaminergic Abnormalities in Human Cocaine Users. Am J
Psychiatry 1999; 156:238-245.
8. Solhkhah, R; Wilens, T;
Pharmacotherapy of Adolescent Alcohol and Other Drug Use Disorders. Alcohol
Health and Research World. Vol. 22, No.2, 1988: 122-125.
10. Graham, A; Schultz; T.
Principles of Addiction Medicine. American Society of Addiction Medicine, Inc.
1998.
Koob,G Roberts, A; Neurocircuitry Targets in Reward and Dependence.
Chapter 6 73-82.
11. Joseph, R. Neuropsychiatry,
Neuropsychology, and Clinical Neuroscience: Emotion, Evolution, Cognition,
Language, Memory, Brain Damage, and Abnormal Behavior. Section III. The Limbic System Williams & Wilkens 1996.
12.Caroll Pharm Iv for Alchohol and
Cocaine Abusing Adults. American J of Addiction 2:77-79 1993.
13.
Navarjo Ca, Sellers Em, Lawvin Mo. Modulation of ethanol intake by
Serotonin Reuptake inhibitors . J. Clinical Psychiatry 47: 16-22. 1986.